- Process Optimization
Enhance Efficiency, Maintain Compliance
- Validation-Based Process Improvement
Optimize Operations Without Sacrificing Compliance


Your Trusted Partner in Validation Excellence
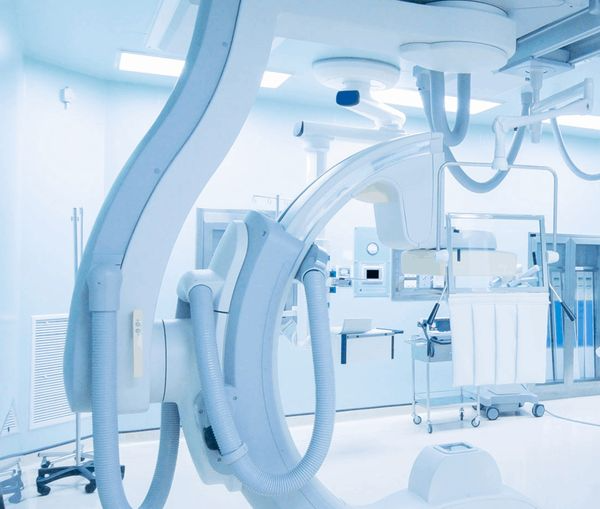
Comprehensive cleanroom certification and equipment validation ensuring safety, quality, and regulatory compliance for critical pharmaceutical and medical facilities.

- Efficiency Improvements & Cost Reduction
Costs Through Smarter Validation Strategies
Validation and compliance don’t have to be expensive or disruptive. Our process optimization team identifies opportunities to reduce downtime, lower costs, and improve efficiency without compromising quality or regulatory standing. We evaluate your current monitoring frequency, qualification schedules, and maintenance procedures to identify areas where optimization can deliver savings. Perhaps you’re conducting unnecessary testing, maintaining overly conservative intervals, or using inefficient procedures. We develop data-driven recommendations reducing costs while maintaining validation. Our clients typically reduce validation and compliance expenses by 15-30% while simultaneously improving operational metrics and regulatory confidence. These improvements fund themselves through cost savings while enhancing your facility’s competitive advantage.
Get Trusted
Validation Experts,
Process Made
Simple
- Equipment & System Modernization
Upgrade Systems While Maintaining Compliance
Older equipment and systems often require extensive resources to keep compliant. Our optimization services evaluate whether modernization—upgrading to newer, validated equipment—offers advantages over maintaining legacy systems. We conduct feasibility analyses comparing the cost and timeline of keeping equipment qualified versus upgrading to more efficient systems. Modern equipment often qualifies faster, requires fewer resources to maintain, and delivers better performance. We provide detailed business cases comparing options, guiding your investment decisions. We’ve helped facilities evaluate equipment lifecycle decisions, planned strategic upgrades, and executed transitions while maintaining continuous operations. The right equipment investment, made strategically with proper validation planning, often delivers better compliance and operational results than maintaining aging systems.